Anaemia in Renal Disease
Renal anaemia is a normochromic normocytic anaemia that increases in severity as renal function declines. There are a number of explanations for anaemia in patients with renal failure, but deficiency of erythropoietin (EPO) is usually dominant when patients are nearing end-stage. EPO is a hormone produced by the kidneys in response to an anaemic hypoxic stimulus; it acts to correct anaemia by stimulating red cell production in the bone marrow. Anaemia due to EPO deficiency can occur with any stage of CKD, but does not usually develop until eGFR is less than 30ml/min/1.73m2 (<45/min/1.73m2 in patients with diabetes) and worsens with declining renal function.1
EPO replacement therapy was developed in the 1980’s, and its use has now become widespread. It has revolutionised the management of renal anaemia, which was previously dependent on repeated blood transfusions and use of androgenic steroids. The majority of patients who require renal replacement therapy will require EPO replacement; the one notable exception to this rule is patients with polycystic kidney disease, who often do not develop EPO deficiency.
However, patients with renal disease are susceptible to anaemia for many reasons, not only EPO deficiency.
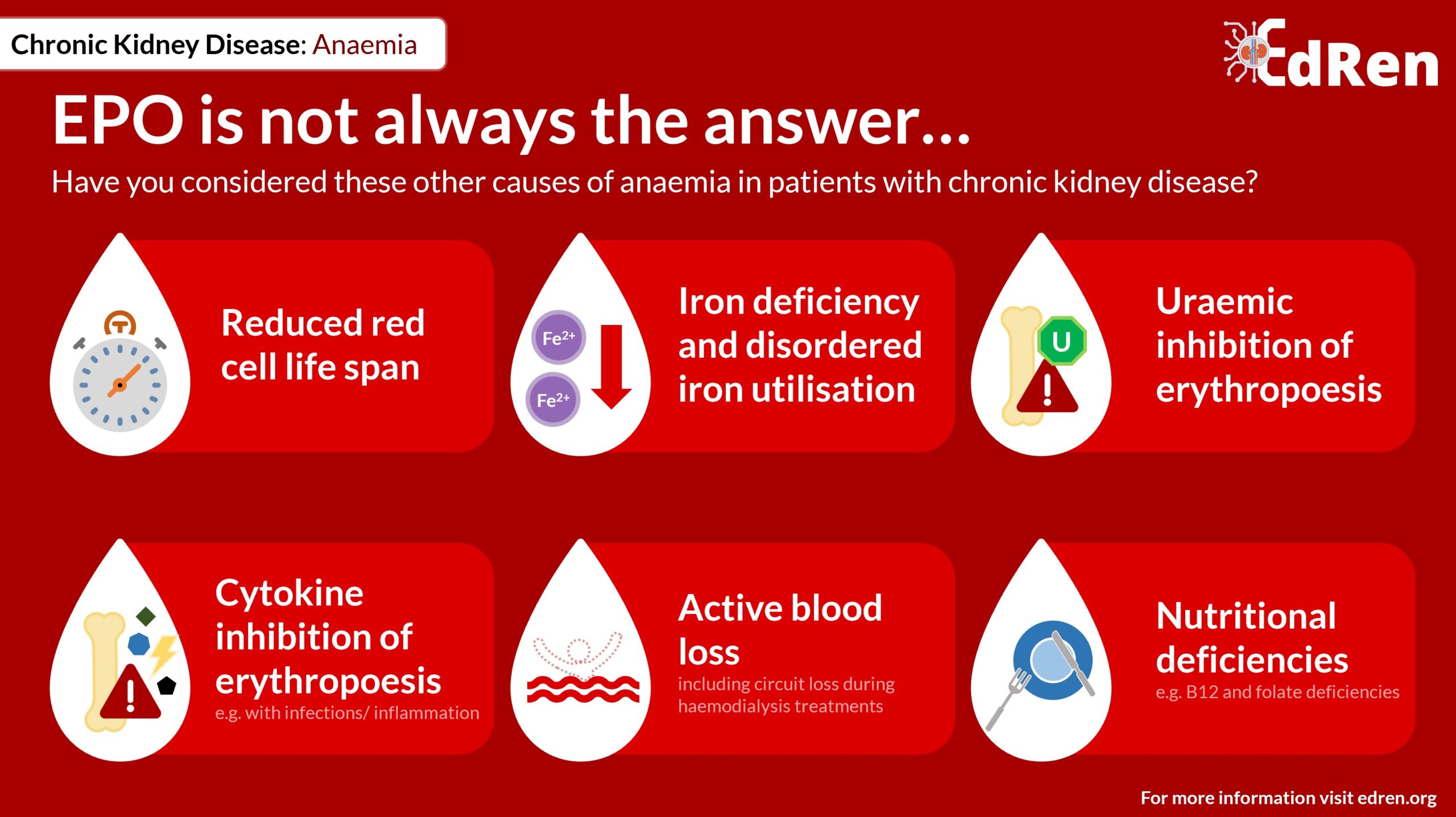
Other causes of anaemia in patients with renal failure include:
- Reduced red blood cell lifespan
- Iron deficiency and disordered iron utilisation
- Uraemic inhibition of erythropoiesis
- Cytokine inhibition of erythopoiesis, e.g. during infections and in inflammatory disorders
- Active blood loss (including circuit loss during haemodialysis treatments)
- Nutritional deficiencies, e.g. B12 and folate deficiency
Without effective treatments anaemia can be very severe and an association exists between low haemoglobin and risk of morbidity and mortality ESRF2. Improving haemoglobin by use of erythropoiesis-stimulating agents (ESAs) results in improvements in exercise tolerance, quality of life, cognitive function, nutrition, and cardiac status (including reduce left ventricular hypertrophy and dilatation).3-4 However, whilst you might assume that restoring haemoglobin to within a physiological range would be most beneficial, studies have not supported this practice due to an increased risk of cardiovascular events.5-6 The exact mechanism of this is unclear, and there is some suggestion that it may be the high doses of EPO used to achieve a normal haemoglobin, rather than the absolute haemoglobin in itself which may confer the increased risks.7 However, given these concerns, recommended target haemoglobin for patients receiving ESAs is therefore low-normal, usually between 100 and 120g/L.8
Baseline Investigations
In accordance with the Renal Association Clinical Practice Guideline 2017 we would recommend the following investigations for patients with CKD who are found to be anaemic; these are similar to those investigations you would perform for patients without CKD.
| Full blood count |
|
| Iron status |
|
| Haematinics |
|
| Inflammation |
|
| Haemolysis screen |
|
| Exclude myeloma / paraproteinaemia |
|
Based on the results of the above investigations, a decision can me made as to whether or not anaemia is related purely to EPO deficiency, or whether there are additional contributory factors which require alternative treatment. Initiation of ESA replacement therapy is done under the supervision of a consultant nephrologist.
References
- Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia. The Third National Health and Nutrition Examination Survey (1988–1994) Archives of Internal Medicine. 2002;162(12):1401–1408.
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis 28 : 53 –61, 1996.
- Canadian Erythropoietin Study Group: Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. BMJ 300 : 573 –578, 1990.
- Revicki DA, Brown RE, Feeny DH, et al. Health-related quality of life associated with recombinant human erythropoietin therapy for predialysis chronic renal disease patients. American Journal of Kidney Diseases. 1995;25(4):548–554.
- Singh AK, Szczech L, Tang KL, et al. Correction of anaemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006 Nov 16; 355(20):2085-98.
- KDOQI clinical practice guideline and clinical practice recommendations for anaemia in chronic kidney disease, 2007 update of haemoglobin target. Am J Kidney Dis 2007; 50(3): 471–530.
- Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved haemoglobin outcomes. Kidney Int. 2008 Sep; 74(6):791-8.
- Locatelli F, Aljama P, Canaud B, et al. Anaemia Working Group of European Renal Best Practice (ERBP). Target haemoglobin to aim for with erythropoiesis-stimulating agents: a position statement by ERBP following publication of the Trial to Reduce cardiovascular Events with Aranesp Therapy (TREAT) study. Nephrol Dial Transplant. 2010; Sept 25(9):2846-50.