Contents
PKD and the use of Tolvaptan
This page is mainly aimed at staff. We have additional information for patients and others at edren.org/gotpkd, and other further info. We also have a page on how to evaluate patients who are found to have kidney cysts (which may be simple, malignant or due to an inherited cystic disease).
Tolvaptan
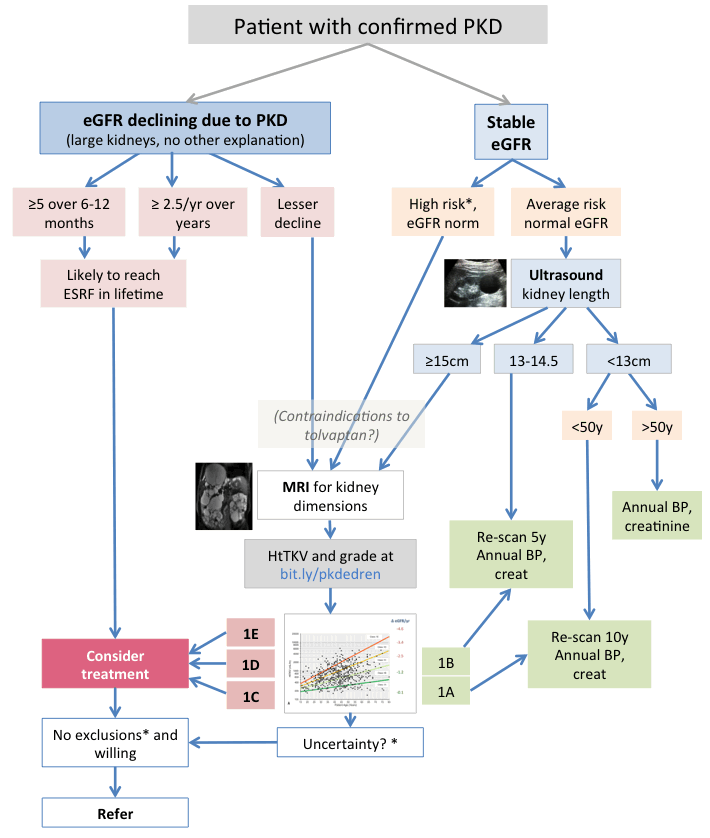
Tolvaptan is an ADH receptor antagonist. It has been shown to reduce rate of cyst growth and GFR decline in PKD, and since late 2015 is licensed for use in the UK and other countries for patients with PKD who are at high risk of progression. Our criteria for this, and other pathway details, are subject to change.
Assessing risk of progression is based primarily on:
- GFR decline – though this is a late sign, it often occurs over a decade or more. Where you’re sure that PKD is the cause (very big kidneys with no other explanation) the criteria are (eGFR in ml/min/1.73m2)
- A fall in eGFR of 5 or more over 6-12 months (confirmed by more than one test, ensuring no other explanation)
- A fall in eGFR of 2.5 or more per year over a longer time period
- Kidney volume normalized for height (HtTKV), matched to age. Bigger kidneys in younger people are more likely to lead to loss of kidney function. Estimating kidney volume is described below. With this criterion it is hoped to identify and treat patients at high risk before their GFR falls. But it poses the question of how to identify and screen all who may be in this group.
- Other factors, including genetic information, family history of early ESRF, early onset of symptoms or hypertension, easily palpable kidneys in young people, are useful in drawing attention to the need to estimate GFR and kidney size.
Protocol
March 2016 (additional detail in protocol document at foot of page)
* see above or below: Other risk factors – imaging uncertainty – tolvaptan exclusion criteria
Note that several criteria cited here are experimental, including ultrasound sizes to request MRI, and re-scan intervals. Progress will be monitored and recommendations modified accordingly. Equally it may be justifiable to vary these criteria in particular circumstances.
Estimating kidney volume
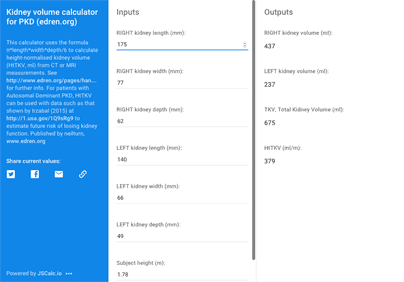
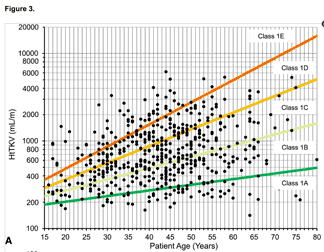
Kidney volume can be a useful predictor of risk, IF kidneys are reasonably evenly enlarged by many small to medium sized cysts* and there are not other important factors operating. You need measurements of subject height as well as kidney dimensions by MRI or CT – enter these into the online TKV calculator (ignore the login box there, it works without) and then use the derived HtTKV with data such as that shown by Irzabal (2015) (see figure) to estimate future risk of losing kidney function.
How do I get those measurements? See Downloads at the foot of this page.
 |
 |
The calculator uses the formula length*width*depth*π/6 to calculate HtTKV, height-normalised kidney volume (ml) from CT or MRI measurements.
Type your values into Inputs and results will automatically populate the Outputs. You can also save or share your values and results from there. The email link is the only useful one.
* Kidneys should be diffusely enlarged by many cysts ‘where all cysts contribute similarly to TKV’ – in other words, a few giant cysts shouldn’t make a substantial component, and there shouldn’t be substantial areas without cysts, or atrophied. Example illustrations of such ‘type 2’ patterns are given in Irazabal 2015 (supplementary data 2). But it is important to note that even when conditions are optimal, estimations of risk made using these techniques are likely to give imprecise estimates of risk for individuals. Actual progression may be much shorter or much longer, depending on cofactors that we may only partially understand, or currently be unaware of.
Exclusions to taking tolvaptan
- GFR <30ml/min/1.73m2 is an exclusion to commencing therapy in current EMA, NICE, SMC guidance. (In England only, NICE guidance also states that eGFR>90 is an exclusion). However if decline rate is very slow, you could still usefully lengthen time to ESRF.
- <18 years old
- Pregnancy
- Requirement for diuretics is a relative contraindication; use caution
- Not able to drink freely
- High risk of AKI from other etiologies. ACEi therapy is not a contraindication in healthy patient who can follow sick day rules
- Liver disease
- Not willing to comply with monitoring.
Other aspects of management
We recommend adding a statin in high-risk patients. Atorvastatin 20mg daily is the NICE-recommended therapy for CKD. Other issues are considered in the short Edinburgh protocol that is downloadable at the foot of this page.
Further info
- Got PKD? – landing page for patients, GPs and others seeking info
- PKD – advanced info for patients – including about Tolvaptan (Edren Info)
- Inherited kidney diseases – very concise for students (Edren textbook)
- PKD on Radiopaedia – excellent images, good background info, plus links to browse
- A patient with big kidneys (Virtual Renal Clinic) – take this simple test case