
You are a doctor working in the paediatric emergency department when a baby who has been vomiting repeatedly all day, is brought in by their parents.
Venous access is gained and bloods taken. As part of this a blood gas is taken.
Without looking at the results what do you might think this baby might be at risk of in terms of acid base abnormality?
Remember vomit is essentially hydrochloric acid with food mixed in. Therefore profuse vomiting will lead to hydrogen ion loss in itself and therefore a possible metabolic alkalaemia.
Starvation – if the baby is unable to manage to keep down any milk can lead to ketoacidosis.
If the baby is septic from whatever is causing the vomiting she could have a lactic acidosis.
The results of the blood gas are detailed below.
| Her results were: [H+] = 30 nmol/L PH = 7.52 |
What is his acid base disturbance in this case?
(Choose one of these and then click to see if you are correct)
Metabolic acidaemia presents with high [H+], significantly low [HCO3-] and low PaCO2. None of these are present in the above blood gas.
He has a metabolic alkalaemia due to loss of [H+] in the vomit. Although there is a more complete answer
He has a metabolic alkalaemia due to loss of [H+] in the vomit with a small compensatory raise in PCO2.
Respiratory alkalaemia usually occurs due to hyperventilation of the lungs and results in significant low PaCO2 and a fall in [H+]
Typical respiratory acidaemia presents with increased PaCO2 and compensatory increased [HCO3-].
If this Baby were to keep vomiting without intervention what complication might he be susceptible to?
Think about this answer and when you are ready click reveal
Our bodies require very careful balances in our pH (7.35-7.45). If there is deviation from this in either direction, it can affect channels, enzymes and metabolic processes throughout the body.
For example where an individual has a metabolic alkalemia, in particular if severe, (pH>7.6) it can lead to life-threatening seizures and ventricular arrhythmias.
The baby is floppy and generally not well. He is not making attempts to gurgle or make any noise apart from the occasional sniffle. He is alert.
You realise that this baby is sick. What would you do to manage this patient?
The old adage two heads are better than one generally can be applied when you feel stuck or out of your depth. With young people and babies they can go from okay to very unwell very quickly so having someone else there in a scenario like this can help.
This baby’s potassium is 2.6. If this is further diluted or sustained it could lead to dangerous arrhythmias. This should be replaced.
In any patient with vomiting, rehydration is key. This baby is hypernatraemic and it is likely that this will be resolved simply be rehydration. Remember sustained hypernatraemia can lead to confusion, seizures and death.
No this patient’s bicarbonate is already high so giving more iatrogenically is only going to worsen the situation.
Whilst important to manage the issue in front of us there must be a reason why this baby is vomiting so much, whether this is a delayed presentation of pyloric stenosis, reflux or a gastrointestinal infection, finding the cause will help prevent this from occurring again.
Which of the following is another cause of metabolic alkalaemia?
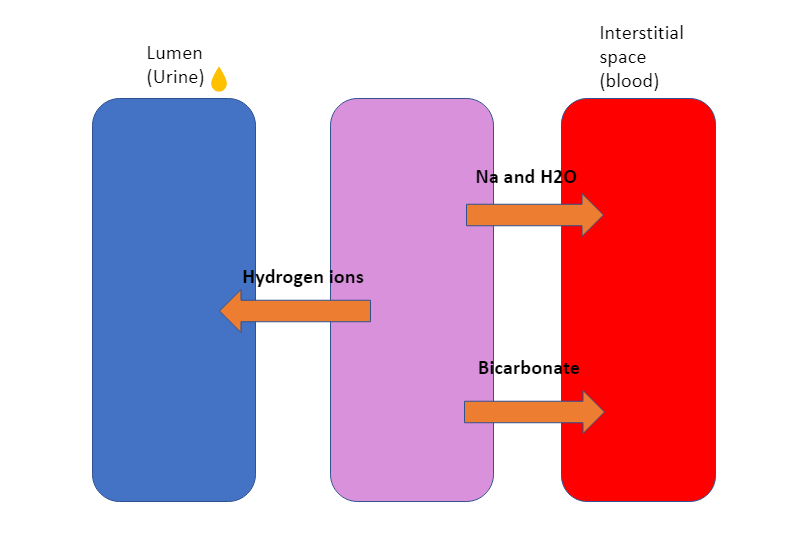
Patients with nephrotic syndrome can get a metabolic alkalosis due to renal hydrogen ion loss and increased tubular bicarbonate absorption. This is due to increased sodium and water resorption. Due to Na/ Bicarbonate co-transporter there will be increase bicarbonate absorption (Alkali). Due to Na/H transporter there is a loss of hydrogen ions (Alkali). This gives a net gain of alkali in the blood therefore metabolic alkalosis.
Carbon dioxide and water is breathed out forming carbonic acid. In hyperventilation more carbon dioxide and water is breathed out than oxygen in. This results in a net loss of acid resulting in a respiratory alkalosis. This is seen in pain, panic attacks and type 1 respiratory failure.
Sepsis can produce a lactic acidosis (Metabolic acidosis). This is thought to be a mixture of increased oxygen demand due to increased metabolic state leading to increased pyruvate (see the Krebs Cycle for further explanation) and tissue hypoperfusion. Both of these lead to raised serum lactate and thus a metabolic acidosis.
Type 2 respiratory failure can occur in conditions such as COPD, neurological dysfunction eg Guillain – Barre syndrome, muscular dystrophy, opiate overdose. It causes carbon dioxide gas trapping and thus leads to Respiratory acidosis.
Think of some further causes of metabolic alkalosis before revealing some other causes listed below.
| Decreased concentration of hydrogen ions | Direct causes of raised bicarbonate |
| – GI losses – vomiting and diarrhoea – Renal losses Nephrotic syndrome Diuretics – Thiazide and loop – Cirrhosis – Heart failure | – Iatrogenic Sodium bicarbonate tablets Citrate in Haemofiltration Citrate in blood transfusions Excess liquorice consumption – Cushing’s – Potassium depletion |
The baby is diagnosed with viral gastroenteritis and improves with some targeted fluid resuscitation. After 24 hours there is no further concerns and she is discharged to the care of her parents.
Well done you have now completed this case.
